From The University of Tulsa
33. Chapter 14: Radical Reactions. S. R. Hussaini (2023), vol. 55, p 597–632. In Organic Reaction Mechanisms 2019: An annual survey covering the literature dated January to December 2019; M. G. Moloney Eds; Wiley.

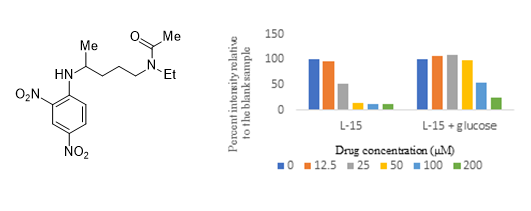
32. Chloroquine-based mitochondrial ATP inhibitors. Z. Wang, R. J. Sheaff, and S. R. Hussaini, Molecules, 2023, 28, 1161.
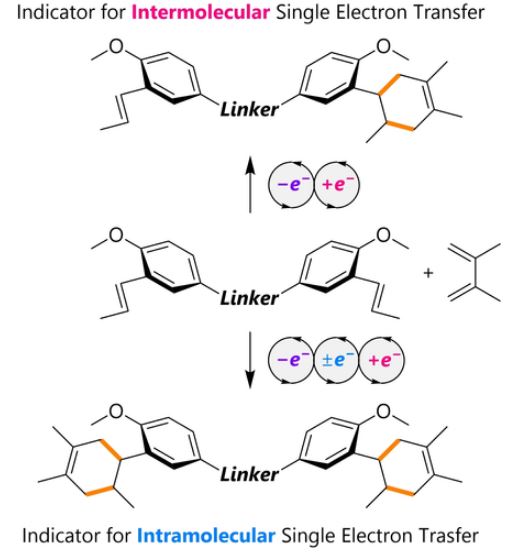
31. Probing electron transfer events in radical cation cycloadditions: intramolecular vs. intermolecular single electron transfer. S. Tanami, S. R. Hussaini, Y. Kitano, K. Chiba and Y. Okada, European Journal of Organic Chemistry, 2022, e202201023.
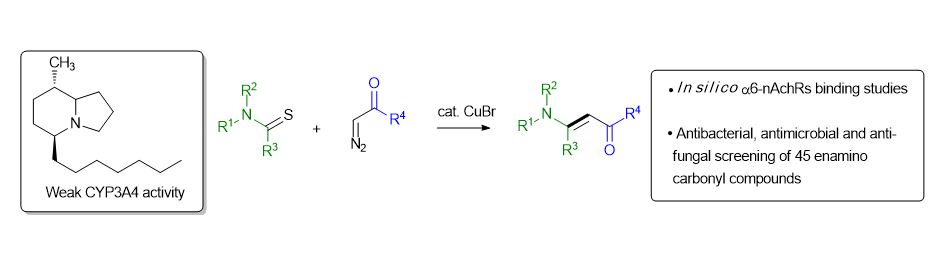
30. Coupling of acceptor-substituted diazo compounds and tertiary thioamides: synthesis of enamino carbonyl compounds and their pharmacological evaluation. J. Secka, A. Pal, Francis A. Acquah, B. H. M. Mooers, A. B. Karki, D. Mahjoub, M. K. Fakhr, D. R. Wallace, T. Okada, N. Toyooka, A. Kuta, N. Koduri, D. Herndon, K. P. Roberts, Z. Wang, B. Hileman, N. Rajagopal and S. R. Hussaini, RSC Advances, 2022, 12, 19431.
29. Total Synthesis of Decahydroquinoline Poison Frog Alkaloids ent-cis-195A and cis-211A. T. Okada, N. Wu, K. Takashima, J. Ishimura, H. Morita, T. Ito, T. Kodama, Y. Yamasaki, S. Akanuma, Y. Kubo, K. Hosoya, H. Tsuneki, T. Wada, T. Sasaoka, T. Shimizu, H. Sakai, L. P. Dwoskin, S. R. Hussaini, R. A. Saporito and N. Toyooka, Molecules, 2021, 26, 7529.

28. Simulations of promising indolizidine–alpha6beta2 nicotinic acetylcholine receptors. F. A. Acquah, M. Paramel, A. Kuta, S. R. Hussaini, D. R. Wallace and B. H. M. Mooers, International Journal of Molecular Sciences, 2021, 22, 7934.
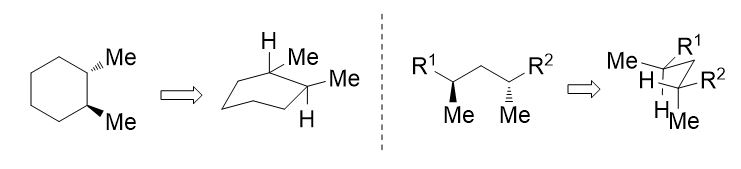
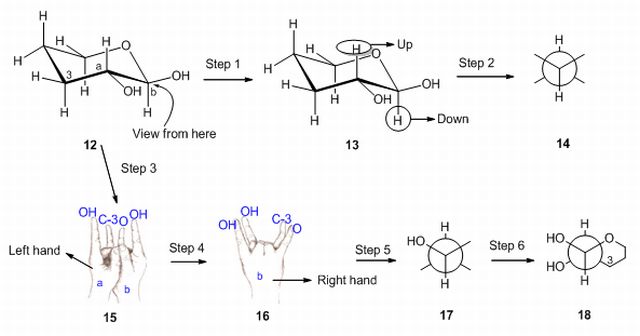
27. Drawing methods for lowest energy boat and pentane conformations. S. R. Hussaini and J. Secka, Journal of Advances in Education, 2021, 6, 1-8.
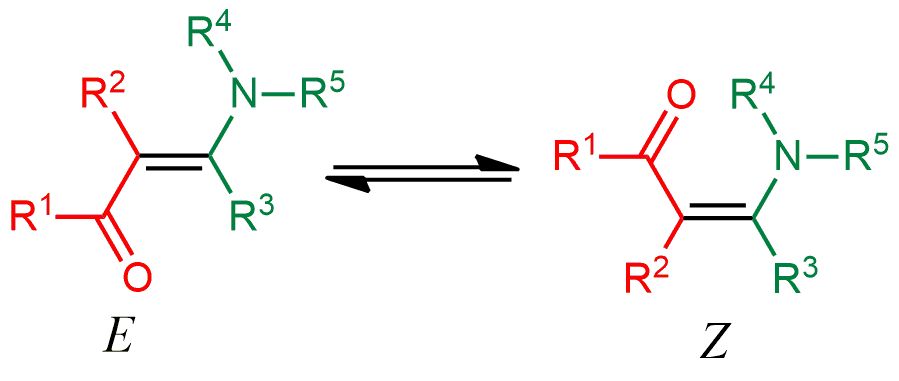
26. Application of NMR spectroscopy for the detection of equilibrating E–Z diastereomers. S. R. Hussaini, A. Kuta, A. Pal, Z. Wang, M. A. Eastman and R. Duran, ACS OMEGA, 2020, 5, 24848-24853.
25. Chapter 1: Reactions of aldehydes and ketones and their derivatives. S. R. Hussaini (2020), vol. 53, p 1–62. In Organic Reaction Mechanisms 2017: An annual survey covering the literature dated January to December; A. C. Knipe & M. G. Moloney Eds; Wiley.

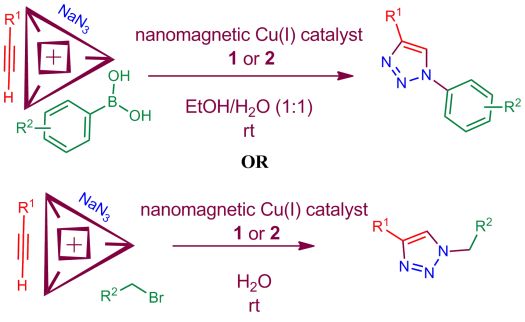
25. Application of two magnetic nanoparticle-supported copper(I) catalysts for the synthesis of triazole derivatives. L. Mohammadi, M. A. Zolfigol, M. Yarie, M. Ebrahiminia, K. P. Roberts and S. R. Hussaini, Research on Chemical Intermediates, 2019, 45, 4789–4799.
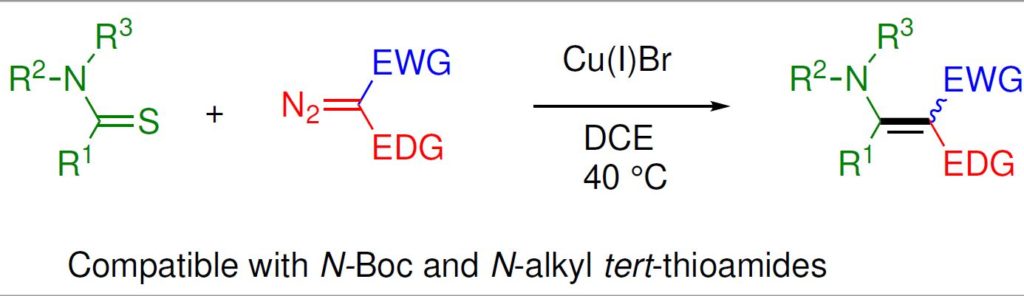
23. Copper-catalyzed coupling of thioamides and donor/acceptor-substituted carbenoids: Synthesis of enaminoesters and enaminones. A. Pal and Syed R. Hussaini, ACS OMEGA, 2019, 4, 269–280.
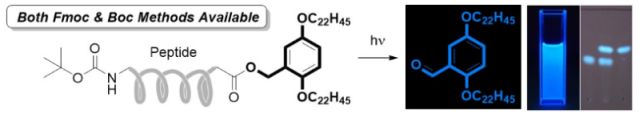
22. Photo-triggered fluorometric hydrophobic benzyl alcohol for soluble tag-assisted liquid-phase peptide synthesis. H. Wakamatsu, Y. Okada, M. Sugai, S. R. Hussaini and K. Chiba, Asian Journal of Organic Chemistry, 2017, 6, 1584–1588.
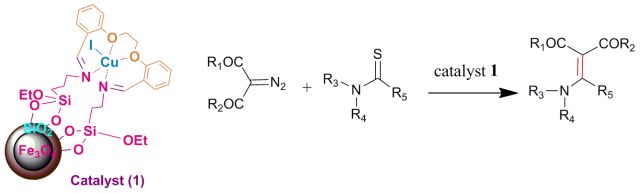
21. A copper(I)-complexed magnetic nanoparticle catalyst for enaminone synthesis. L. Mohammadi, M. A. Zolfigol, M. Ebrahiminia, K. P. Roberts, S. Ansari, T. Azadbakht, S. R. Hussaini, Catalysis Communications, 2017, 102, 44–47.
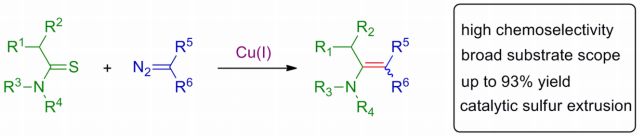
20. Copper-catalyzed chemoselective cross-coupling reaction of thioamides and alpha dizocarbonyl compounds: Synthesis of enaminones. A. Pal, N. D. Koduri, Z. Wang, E. L. Quiroz, A. Chong, M. Vuong, N. Rajagopal, M. Nguyen, K. P. Roberts, S. R. Hussaini, Tetrahedron Letters, 2017, 58, 586–589.
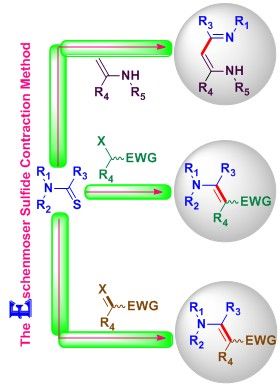
19. The Eschenmoser sulfide contraction method and its applications in the synthesis of natural products. S. R. Hussaini, R. R. Chamala and Z. Wang, Tetrahedron, 2015, 71, 6017–6086.
18. Identification of novel proteasome inhibitors from an enaminone library”, M. Elliott, K. Thomas, S. Kennedy, N. D. Koduri, S. R. Hussaini and R. Sheaff, Chemical Biology & Drug Design, 2015, 86, 322–332.
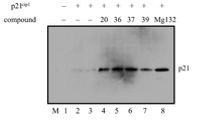
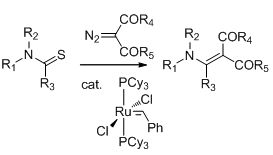
17. Enaminones via ruthenium-catalyzed coupling of thioamides and alpha diazocarbonyl compounds. N. D. Koduri, Z. Wang, G. Cannell, K. Cooley, T. Tsebaot, K. Miao, M. Nguyen, B. Frohock, M. Castaneda, H. Scott and S. R. Hussaini, The Journal of Organic Chemistry, 2014, 79, 7405–7414.
16. Converting chair-like transition states into zig-zag projections: A method of drawing stereochemical structures. S. R. Hussaini, Resonance – Journal of Science Education, 2014, 19 (9), 846–850.
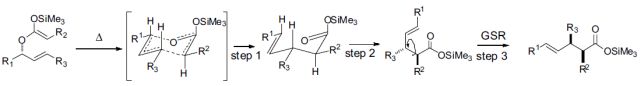
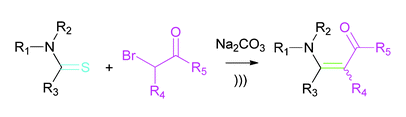
15. Acceleration of the Eschenmoser Coupling Reaction by Sonication: Efficient Synthesis of Enaminones. N. D. Koduri, B. Hileman, J. D. Cox, H. Scott, P. Hoang, A. Robbins, K. Bowers, L. Tsebaot, K. Miao, M. Castaneda, M. Coffin, G. Wei, T. D. W. Claridge, K. P. Roberts and S. R. Hussaini, RSC Advances, 2013, 3, 181–188.
14. Method for the Drawing of Newman Projections: Understanding Newman Projections with the help of hands. S. R. Hussaini, Resonance – Journal of Science Education, 2012, 17 (3), 291–294.
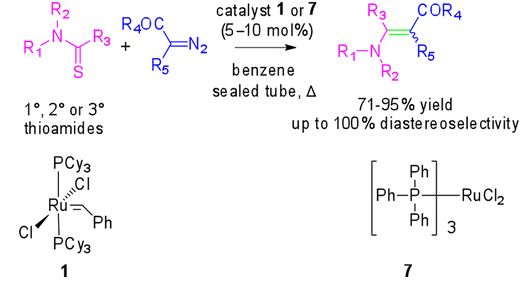
13. Ruthenium catalyzed synthesis of enaminones. N. D. Koduri, H. Scott, B. Hileman, J. D. Cox, M. Coffin, L. Glicksberg and S. R. Hussaini, Org. Lett., 2012, 14, 440–443.
12. Synthesis of stacked-cup carbon nanotubes in a metal free low temperature system. Y. Kimura, J. A. Nuth, N. M. Johnson, K. D. Farmer, K. P. Roberts and S. R. Hussaini, Nanosc. Nanotechnol. Lett., 2011, 3, 4–10.

11. Interconversion of Fischer and Zig-zag Projections: Learning Stereochemistry with the help of Hands. S. R. Hussaini, Resonance – Journal of Science Education, 2010, 15 (4), 351–354. Missing Scheme 1–2.
Earlier Publications
10. Eschenmoser coupling reaction of selenoamides. Synthesis of enamino ester. S. R. Hussaini and G. B. Hammond, ARKIVOC, 2008, xiii, 129–136.
9. 2,5-Disubstituted pyrrolidines: synthesis by enamine reduction and subsequent regioselective and diastereoselective alkylations. S. R. Hussaini and M. G. Moloney, Org. Biomol. Chem., 2006, 4, 2600–2615.
8. On the Sequence of Bond Formation in Loline Alkaloid Biosynthesis. J. R. Faulkner, S. R. Hussaini, J. D. Blankenship, S. Pal, B. M. Branan, R. B. Grossman and C. L. Schardl, ChemBioChem, 2006, 7, 1078–1088.
7. Regioselective Reduction of ß-enaminoesters. S. R. Hussaini and M. G. Moloney, Synth. Commun., 2005, 35, 1129–1134.
6. Butyrylcholinesterase Inhibitory Lignans from Sarcostemma viminale. V. U. Ahmad, M. Zubair, M. A. Abbasi, F. Kousar, S. A. Nawaz, M. I. Choudhary and S. R. Hussaini, Proc. Pakistan Acad. Sci., 2005, 42, 167–171.
5. cis-Selective Synthesis of 2,5-Disubsituted Pyrrolidines. S. R. Hussaini and M. G. Moloney, Tetrahedron Lett., 2004, 45, 1125–1127.
4. 2,5-Disubstituted Pyrrolidines: versatile regioselective and diastereoselective synthesis by enamine reduction and subsequent alkylation. S. R. Hussaini and M. G. Moloney, Org. Biomol. Chem., 2003, 1, 1838–1841.
3. Matricarin. M. Parvez, V. U. Ahmad, U. Farooq, A. R. Jassbi and S. R. Hussaini, Acta Crystallographica Sec. E, 2002, E58, o324–o325.
2. Eight New Diterpenoids from Euphorbia decipiens. M. Zahid, S. R. Hussaini, M. Abbas, Y. Pan, A. R. Jassbi, M. Asim, M. Parvez, W. Voelter and V. U. Ahmad, Helv. Chim. Acta, 2001, 84, 1980–1988.
1. Flavonoids of Tephrosia purpurea. V. U. Ahmad, Z. Ali, S. R. Hussaini, F. Iqbal, M. Zahid, M. Abbas, N. Seba, Fitoterapia, 1999, 70, 443–445.