Our group is interested in the development of selective C-C bond-forming methods and their application to the synthesis of bioactive compounds. We are also interested in applying our methods to domino sequences.
Presently, we are investigating:
- Efficient methods for the preparation of enaminones
- Enantioselective synthesis of anti-smoking agents
Preparation of Enaminones
Enaminones have long been used as synthetic intermediates in organic synthesis. They can react with electron-rich as well as electron-deficient compounds. Therefore, the development of methods that can provide access to a broad range of enaminones is valuable. We have developed ruthenium- and copper-catalyzed methods for coupling diazocarbonyl compounds with thioamides. The reactions are successful with various Ru(II) and Cu(I) catalysts.1-3 We are using this transformation in conjunction with the epoxidation reaction to prepare epoxy enaminones in a one-pot sequence.
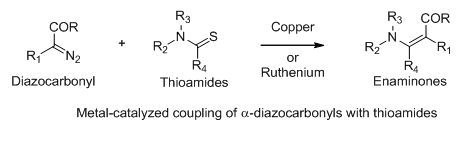
The Eschenmoser coupling reaction condenses a thioamide and an alpha-halocarbonyl compound to give enaminones. We have developed a sonication-accelerated version of the Eschenmoser coupling reaction. Reaction conditions have reduced the reaction time from days to hours.4 Presently, efforts are underway to further reduce the time of reaction to minutes by manipulating the reaction conditions.
Enantioselective synthesis of anti-smoking agents
Smoking is a leading cause of preventable deaths. An effective and safe smoking cessation agent can drastically reduce this loss. Currently available anti-smoking drugs have limited efficacy and high relapse rates. This is why we are conducting an investigation to find more effective agents.
Research is underway to develop an enantioselective method for the synthesis of compounds with the required structural features to bind to the a4b2 receptors. These receptors are considered to be responsible for nicotine addiction.
Electrosynthesis
Electrosynthetic methods tend to be clean, versatile, and atom-economical. We are initiating projects to enable regioselective functionalization of heterocycles via electrosynthesis.
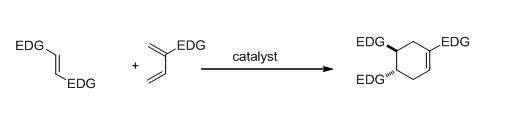
Diels-Alder reactions between electronically mismatched partners
When both the diene and dienophiles are electron-rich, the Diels-Alder reaction requires harsh or specialized conditions/catalysts. We are investigating earth-abundant materials to catalyze such a reaction.
References
- “Ruthenium catalyzed synthesis of enaminones”, N. D. Koduri, H. Scott, B. Hileman, J. D. Cox, M. Coffin, L. Glicksberg and S. R. Hussaini, Org. Lett., 2012, 14, 440–443.
- “Enaminones via ruthenium-catalyzed coupling of thioamides and alpha diazocarbonyl compounds”, N. D. Koduri, Z. Wang, G. Cannell, K. Cooley, T. Tsebaot, K. Miao, M. Nguyen, B. Frohock, M. Castaneda, H. Scott and S. R. Hussaini, The Journal of Organic Chemistry, 2014, 79, 7405–7414.
- “Copper-catalyzed chemoselective cross-coupling reaction of thioamides and alpha dizocarbonyl compounds: Synthesis of enaminones”, A. Pal, N. D. Koduri, Z. Wang, E. L. Quiroz, A. Chong, M. Vuong, N. Rajagopal, M. Nguyen, K. P. Roberts, S. R. Hussaini, Tetrahedron Letters, 2017, 58, 586–589.
- “Acceleration of the Eschenmoser Coupling Reaction by Sonication: Efficient Synthesis of Enaminones”, N. D. Koduri, B. Hileman, J. D. Cox, H. Scott, P. Hoang, A. Robbins, K. Bowers, L. Tsebaot, K. Miao, M. Castaneda, M. Coffin, G. Wei, T. D. W. Claridge, K. P. Roberts and S. R. Hussaini, RSC Advances, 2013, 3, 181–188.